It could be that all the extra carbon dioxide in the atmosphere – the steady increase year on year, reported since 1958, despite all the airplanes grounded through covid it kept on increasing – is a consequence of the oceans breathing out a bit more, and nothing to do with you and me.
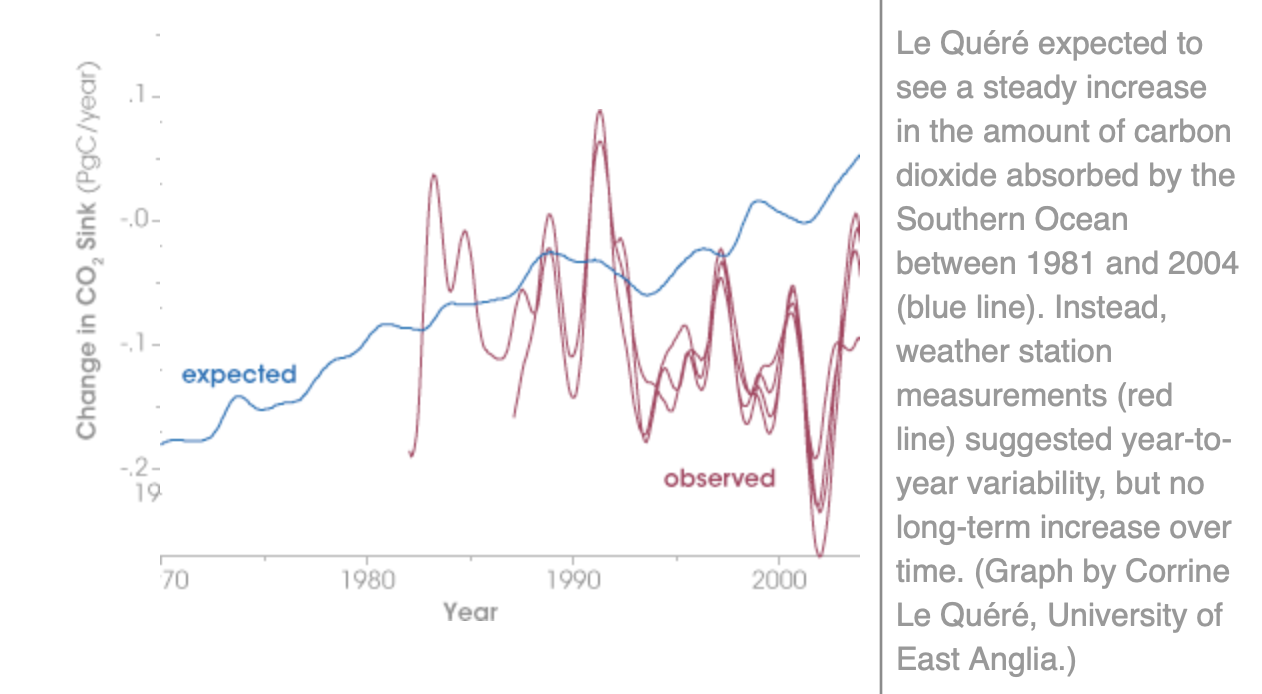
The amount of carbon sequestered in the oceans was meant to increase with the apparently significant increase in atmospheric levels because of industrialisation, except that observations, actual measurements, suggest that there has been no long-term increase. The oceans might be breathing carbon dioxide in, and out, to their own rhythm that has nothing to do with you and me – or all the windfarms.
It could be that mankind is irrelevant to the cycles of ocean warming, that dictates atmospheric levels of carbon dioxide.
The Earth’s oceans contain somewhere in the vicinity of 50 times more carbon than the atmosphere. The global carbon ocean reservoir is about 38,000 gigatons of carbon, while the atmosphere contains only 730 gigatons. In the atmosphere carbon is present as carbon dioxide, which is just 0.04% of the atmosphere by dry weight.
Carbon dioxide is a tiny fraction of the atmosphere, and there is constant exchange of carbon dioxide between the atmosphere and the ocean. This exchange has been described as inhale and exhale, since forever.
The ocean takes up carbon dioxide through photosynthesis by phytoplankton, as well as by simple dissolution, that is carbon dioxide dissolves in water. Carbon dioxide reacts with seawater, creating carbonic acid (H2CO3). Carbonic acid releases hydrogen ions, which combine with carbonate in seawater to form bicarbonate (HCO3-).
The amount of carbon dioxide absorbed by the oceans varies with latitude. At high latitudes/towards the poles the ocean will absorb/store more carbon dioxide and more easily, because of the lower temperatures.
An overall increase in ocean temperatures, especially at the poles where they vary according to long cycles, could mean more exhaling relative to inhaling of carbon dioxide, over the year over the Earth. That is not what the dominant paradigm has theorised. But it is what the measurements show.
Ocean temperatures also vary seasonally. Concentrations of bicarbonate peak in surface seawaters in April at the latitude of Hawaii, which is also the time of year when atmospheric carbon dioxide is highest as measured at Mauna Loa. Atmospheric concentrations of carbon dioxide vary seasonally, as does the sea surface temperature, at the latitude of Hawaii, which is the main site for monitoring global change in atmospheric levels of carbon dioxide.
I will be discussing all of this with Sydney University Professor Ivan Kenney on 25th February, by Zoom. There will be just 100 places if you want to be a part of this discussion, so make sure you are registered for my Mail chimp specifically tick the box ‘New Theory of Climate’ here: https://jennifermarohasy.com/subscribe/
This zoom is part 2 of a discussion I began with Bill Kininmonth last year, more information about that here,
Ivan Kennedy is an emeritus professor in the School of Life and Environmental Sciences at the University of Sydney. His 2023 research paper – entitled ‘The Seasonal Keeling Oscillation in Atmospheric pC02 is Caused by Variation in Seawater Surface Temperature’ published in the journal Examines in Marine Biology and Oceanography – will be distributed to those who register for the zoom a few days before the discussion.
Seasonal variability in atmospheric levels of carbon dioxide and long term trends will be the focus of this discussion with Ivan planned for Tuesday 25th February at noon Brisbane time (Australian Eastern Standard Time, AEST – an hour later in Sydney because of daylight saving and the day before in Texas because of datelines etc.). The link will be in the Mailchimp, for those subscribed to ‘New Theory of Climate’, and I will send it out next Tuesday. That is the plan.

 Jennifer Marohasy BSc PhD is a critical thinker with expertise in the scientific method.
Jennifer Marohasy BSc PhD is a critical thinker with expertise in the scientific method.

This is good thinking. The oceans are huge carbonated drinks, open a hot and a cold carbonated drink, the vapor pressure of CO2 or any gas that is dissolved in water is a function of the temperature of the water and the amount of the gas that is dissolved in the water.
Excellent work, as usual. Thanks Jennifer
Scientists baffled by cooling Atlantic, its not in the script.
‘Scientists at the National Oceanic and Atmospheric Administration (NOAA) found that early 2024, from February to March, saw the strongest Atlantic “warm event” since 1982, with temperatures over 30 degrees Celsius, or 86 degrees Fahrenheit.
‘Since then, from June until now, the Atlantic has undergone a rapid reversal, switching from anomalous heat to a rapidly dropping temperature with unprecedented speed.
‘The sudden cooling trend is baffling scientists.
“Never before in the observed record has the eastern equatorial Atlantic swung so quickly from one to another extreme event,” said Franz Tuchen, writing for NOAA.’ (The Maine Wire)
I’ve been saying that for some 20 years.
The issue then turns to the ice core record which must simply fail to adequately record large scale ocean induced short term variations in atmospheric CO2.
Old news. “The Atlantic is not cooling as a whole, only its equatorial section is experiencing rapid cooling between March and July 2024. Almost the entire surface of the Atlantic Ocean has been warming by 0.5 to 0.75°C per century since 1854.”
“Earlier this month, we wrote about a pocket of cooler-than-average surface waters that had emerged near the equator in the eastern Atlantic in June and July. The localized cool spot was interesting because if it had continued at that strength through August, it would be classified as an Atlantic Niña—a natural pattern of warm-cool swings in the eastern equatorial Atlantic that affects seasonal climate in the region.
Our plan was to wait give the August data a chance to come in and be analyzed before doing a follow-up post in early or mid-September covering whether the event formed or fizzled, what the potential influences could be on seasonal temperature and precipitation in the region, as well as some background on what we know and don’t know about why these events occur and whether human-caused climate change is expected to affect the pattern.
That post is still coming, but we are squeezing in a quick extra post because there has been a lot of interest in the event, as well as a bit of…let’s call it over-interpretation…in some corners of the internet (as well as the Climate.gov webmail inbox) that we want to tamp down if possible.”
By Rebecca Lindsey, Franz Philip Tuchen (Cooperative Institute For Marine And Atmospheric Studies), AND Sang-Ki Lee Published August 28, 2024
https://www.climate.gov/news-features/event-tracker/four-things-know-about-possible-atlantic-nina
Stephen, there seems to be a smoothing of the CO2 in ice cores by diffusion.
Thanks Karen, most importantly the AMOC has remained stable over the last 70 years and it maybe linked to a positive AMO.
Terhaar and the team relied on new data from the Coupled Model Intercomparison Project (CMIP), climate-earth models produced by the World Climate Research Program.
‘The researchers looked at a different measure: air-sea heat fluxes, which is the exchange of heat from the ocean to the atmosphere.
‘When the AMOC is stronger, more heat is released from the ocean to the atmosphere over the North Atlantic.’ (Woods Hole)
And, just for the entertainment of divers and tourists, in “warmer” waters, corals sequester a LOT of “Carbon” in reef building.
This appears to have been going on for some time, witness the vast expanses of “limestone beds around the globe. This also indicates that LAND levels have been rising and falling for quite a while, too. (And skating about laterally).
An interesting map:
https://www.researchgate.net/profile/Alice-Latinne/publication/256461634/figure/fig1/AS:297858646462468@1448026443794/Limestone-karsts-in-Southeast-Asia-dark-grey-and-distribution-of-Southeast-Asian.png
Also; enormous beds of Marble at several thousand feet above current sea level in Italy. (Carrara)? How far down did that lot have to be subducted, and heated and compressed, before being shoved skywards, again. See also North Queensland.
The planetary regime appear to NOT be set in aspic.
When the North Atlantic Oscillation is positive the seas are rougher and there is more upwelling, liberating more CO2 from the depths. The 1990s are a standout.
https://chaac.meteo.plus/en/climate/nao-index-monthly.png
The Pacific Decadal Oscillation, in its negative phase, also produces an increase in CO2 venting.
Corinne Le Quere is alarmed, why aren’t you Jenn?
“The Southern Ocean around Antarctica is so loaded with carbon dioxide that it can barely absorb any more, so more of the gas will stay in the atmosphere to warm up the planet. Researcher Corinne Le Quere said: “We thought we would be able to detect these only the second half of this century, say 2050 or so.” But data from 1981 through 2004 show the sink is already full of carbon dioxide. “So I find this really quite alarming,” she added.”
The Southern Oceans inhales CO2 during the Austral summer and exhales over the winter.
https://climate.nasa.gov/news/3136/nasa-supported-study-confirms-importance-of-southern-ocean-in-absorbing-carbon-dioxide/
They say the system works well and there is no acidification.
GCMs worthless.
https://notrickszone.com/2025/02/11/new-study-todays-climate-models-do-not-agree-with-reality-and-thus-their-usefulness-is-doubtful/
Global weirding is trending.
‘CSIRO coastal oceanographer Ron Hoeke says it takes a lot of research to pin down the cause of a particular event, and usually, it’s neither 100 per cent due to climate change, nor 100 per cent due to natural climate variability.
“It’s some mix of the two, generally — because we’ve got this underpinning variability and almost pseudo-randomness in the natural Earth system,” he says. (SBS)
Emerging CO2 sink in Antarctic waters.
http://www.co2science.org/articles/V13/N49/B3.php
I don’t have words. But let’s suppose ocean is not a net sink. So where is all the anthropogenic CO2 going to? I don’t understand your claim.
I’d suggest the ocean net sink could be larger or smaller than estimated now, but not negative.
Which I can’t find any description.
Since CO2 is highly soluble in water, the part above the equilibrium concentration dissolves when the moisture condenses. The pH of rain is therefore 5.6. 66% of the Earth is covered by clouds. Water in the atmosphere is exchanged every 10 days. So carbon is mainly transferred from the atmosphere to the ocean through rain.